By Gerhard Knothe
ARS, USDA, NCAUR, Peoria, Illinois 61604
(Address correspondenceat NCAUR, ARS, USDA, 1815 N. University St., Peoria, IL 61604. E-mail: knothegh@mail.ncaur.usda.gov)
Presented in part at the 89th AOCS Annual Meeting & Expo, Chicago, IL, May 1998.
JAOCS, Vol. 77, no. 5 (2000)
Abstract
Biodiesel is a promising alternative diesel fuel obtained from vegetable oils, animal fats, or waste oils by transesterifying the oil or fat with an alcohol such as methanol. In an extension of previous work, fiber-optic near infrared spectroscopy was used to quantitatively monitor the transesterification reaction (6-L scale) of a vegetable oil (soybean oil) to methyl soyate. The results were correlated with 1H nuclear magnetic resonance spectroscopy. The method described here can be applied to the transesterification of other vegetable oils.
Paper no. J9483 in JAOCS 77, 489-493 (May 2000).
Key Words: Biodiesel, fiber-optic probe, fuel quality, near infrared spectroscopy, nuclear magnetic resonance, soybean oil, transesterification, vegetable oils.
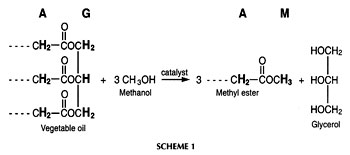
The transesterification of a vegetable oil or animal fat with a monohydric alcohol such as methanol in the presence of a catalyst (usually a base such as NaOH or KOH) affords the corresponding monoalkyl esters [see Scheme 1, in which the protons (methyl ester protons in combination either with a-CH2 or glyceridic protons) used for monitoring the reaction by 1H nuclear magnetic resonance (NMR) spectroscopy are highlighted and also indicated by the letters A (a-CH2 protons), G (glyceridic), and M (methyl ester)]. These esters, especially the methyl esters, have significant potential as alternative diesel fuel (biodiesel) both in neat form and blended with conventional diesel fuel (1, 2). Regulated fleets as well as mining and marine markets are targets for biodiesel commercialization in the United States (3). However, successfully commercializing biodiesel in the future depends on a variety of parameters. One of these parameters is fuel quality as defined in a provisional American Society for Testing and Materials (ASTM) biodiesel standard (4) and existing standards in some European countries. The amount of contaminants (such as glycerol; mono-, di-, and triglycerides; and alcohol) present in the fuel after posttransesterification purification is a major factor in determining fuel quality. The possible contaminants are limited in biodiesel standards and the rationales for various specifications in fuel standards have been discussed (4, 5). The analysis of contaminants in biodiesel fuel is a major issue influencing commercial success because contaminants can lead to severe operational problems such as engine deposits.
Most analytical procedures for determining biodiesel fuel quality utilize gas chromatography (GC) (6-16). Preseparation of biodiesel and its contaminants by high-performance liquid chromatography (HPLC) prior to GC also has been reported (16). Other analytical methods applied to biodiesel include viscosity (17) and HPLC with density detection (18) or pulsed amperometric detection for determining glycerol (19). 1H NMR spectroscopy was used for determining the yield of the transesterification reaction of rapeseed oil with methanol (20).
Analyses of fatty materials by near infrared (NIR) spectroscopy have become widespread in recent years (21-23). Biodiesel was determined in lubricating oil by fiber-optic Fourier transform infrared spectroscopy (24). Recently, we reported (25) that NIR spectroscopy utilizing a fiber-optic probe presents a possibility for rapid, easy-to-handle, and cost-effective monitoring of the transesterification reaction and of biodiesel fuel quality. Although the analysis of trace contaminants at the levels specified in biodiesel standards is not possible by NIR, this problem can be largely circumvented by an inductive method in which monitoring of the transesterification reaction at various stages shows that the reaction is progressing as desired. The NIR method, which utilizes a convenient fiber-optic probe, is considerably easier and faster to use than GC, making it more suitable for a production situation. This usefulness was demonstrated by a model system in which specified amounts of biodiesel contaminants were deliberately added to methyl soyate (biodiesel) (25). It was shown that two different regions in the NIR spectra of methyl esters and triglycerides (vegetable oil) at 6005 and 4425-4430 cm-1 can be used to quantitate the amount of residual vegetable oil feedstock in the methyl esters (biodiesel).
In the present paper, the previous work on fiber-optic NIR spectroscopy is extended beyond the previously discussed model system (25) to monitoring of a transesterification reaction (6 L scale) in progress. We used the formation of methyl soyate from soybean oil as an example, although the results presented here hold for the transesterification of other vegetable oils. As a means for correlating and cross-checking results with another analytical method, 1H NMR spectroscopy was selected. Two combinations of signals were used in 1H NMR, one of which was reported previously by other authors (20), but both involve the methyl ester protons of the biodiesel product.
Experimental Procedures
Refined soybean oil was obtained from Archer Daniels Midland Co. (Decatur, IL) through Pasquel Institutional Foods (Peoria, IL). Methanol was purchased from Fisher Scientific (Fair Lawn, NJ), and potassium hydroxide was acquired from J.T. Baker Chemical Co. (Phillipsburg, NJ).
Reactions were conducted in a three-necked, 12-L glass reactor (Ace Glass Inc., Vineland, NJ) equipped with a stirrer (Glas-Col, Terre Haute, IN; obtained through Ace Glass Inc.), reflux condenser, and a stopcock attached at the bottom for sample removal. For the reaction, 6 L soybean oil, 1470 mL methanol [mole ratio approximately 6:1; stoichiometry according to Freedman et al. (34)], and 18.13 g potassium hydroxide (1% KOH) were used at a temperature of 45 deg C. Samples (approximately 100 mL) were removed at 5-min intervals by dripping into water or water acidified with HCl. The samples were analyzed after separation of the glycerol phase.
NIR spectra were obtained on a PerkinElmer (Norwalk, CT) Spectrum 2000 spectrometer equipped with a Galileo (Sturbridge, MA) transmission-type fiber-optic probe as described in a previous publication (25). Quantitation methods were developed on a personal computer (Spectrum 2000 and Quant+ software; PerkinElmer), also as described previously (25). 1H NMR spectra were obtained on a Bruker ARX-400 spectrometer (Bruker, Rheinstetten, Germany) operating at 400 MHz (solvent: CDCl3). GC analyses were performed on a Hewlett-Packard (Palo Alto, CA) 6890 gas chromatograph according to published procedure (7) with the exception that a split injector was used.
For quantitation in wt% (the limits on most contaminants in biodiesel are expressed in wt%) by NIR, methods developed previously (25) were used. Densities were 0.87104 for methyl esters and 0.9092 for soybean oil (25).
Results and Discussion
NIR applications similar to the present one have been reported, for example, for determining the transesterification endpoint of a methyl ester with polyethylene glycol (26), in-process analysis of multifunctional esters (27), and monitoring OH absorption bands during esterification (28). In the present work, a vegetable oil, soybean oil, was transesterified on a 6-L scale with methanol and potassium hydroxide to methyl soyate. Reaction progress was monitored by removing a portion of the reaction mixture at predetermined intervals and analyzing by NIR and NMR.
While the NIR spectra of numerous fatty compounds have been known for a while (29-32) [the first report dates more than 40 years ago (29)], the NIR spectra of feedstock (soybean oil) and biodiesel fuel (methyl soyate) with the peak differences enabling quantitation were depicted more recently (25).
Since the quantitation method by NIR was discussed in a previous publication (25), no further discussion will be presented here. In any case, the present work shows that the model system using defined amounts of "contaminants" in biodiesel is applicable to an actual transesterification reaction.
In previous 1H NMR-based monitoring of transesterification of rapeseed oil (20), the yield of methyl esters was determined by the integration values of the protons of the methyl ester moiety (at approximately 3.7 ppm) and the a-carbonyl methylene groups (about 2.3 ppm) found in all fatty compounds. In the present work, both the previous methyl ester protons with a-CH2 protons approach (20) and another NMR signal-based approach, namely, the glyceryl protons of the feedstock and the methyl ester protons of the product, were selected (see Fig. 1 for the 1H NMR spectrum of a progressing transesterification reaction). Note that the glyceryl and the methyl ester are readily distinguishable in NIR (25). No internal standard or calibration of the NMR part of the work is required either with the equations from previous literature (20) or with those developed here for the second NMR approach. Figure 2 is a plot of conversions determined by NIR and both NMR approaches for a transesterification reaction of soybean oil with methanol carried out at 45 deg C for 1 h.
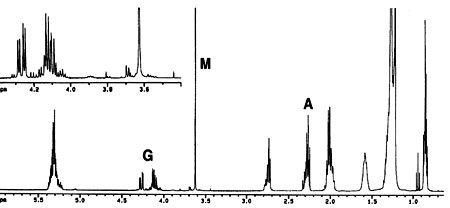
Fig. 1. 1H nuclear magnetic resonance (NMR) spectrum of a progressing transesterification reaction (spectrum recorded after 5 min reaction time at 45 deg C). The letters A, G, and M denote the a-CH 2, glyceridic, and methyl ester protons, respectively (see also Scheme 1).
NMR was chosen for correlation with NIR in the present work for several reasons. First, to the best of our knowledge the glyceryl and methyl ester protons of the feedstock and the methyl esters have not been used previously in monitoring the transesterification reaction and only one previous report (20) exists for monitoring the transesterification reaction by NMR. None of the NMR signals of the selected moieties overlap and glycerol does not interfere as it nearly completely separates from the methyl ester phase before analysis. Second, NMR may be an alternative to other methods when contracting analyses, besides GC, to an analytical laboratory. The generally higher cost of NMR equipment may become less of an obstacle. Third, the GC method (7) currently recommended in the provisional ASTM biodiesel standard is not without problems in our experience. These problems resulted from the column being heated beyond its recommended temperature limit (370 instead of 350 deg C; no mention of a high-temperature column being used) and, in connection with this, ballistic heating from 230 to 370 deg C in 3.2 min (nearly 44 deg C/min as calculated from the given run time). Thus, baseline drifts occurred that interfered with quantitation (especially considering the low contamination levels specified in biodiesel standards), and repeated use at the high temperature eventually causes stronger column bleed. While other GC methods exist, they do not offer any further advantages for simultaneous quantitation of feedstock, methyl esters, and glycerol. Modifying the parameters of the aforementioned GC method (7) to circumvent the problems was not carried out in the present work.
The conversion CME (in %) of vegetable oil (soybean oil) to biodiesel (methyl soyate) can be calculated from the integration values of the glyceridic and methyl ester protons in 1H NMR by Equation 1:
![]()
1ME is the integration value of the methyl ester peak and 1TAG is the integration value of the glyceridic peaks in the triacylglycerides (TAG) of the vegetable oil. The factors 5 and 9 result from the fact that the glyceryl moiety of a triglyceride has five protons and the three methyl ester moieties resulting from one triglyceride molecule have nine protons (see Scheme 1). Figure 1 depicts the 1H NMR spectrum of a transesterification reaction in progress. Note that mono- and diacylglycerides, which are formed as intermediates in the transesterification reaction, exhibit signals of their glyceridic protons in the same region as glyceridic protons of the triacylglycerides in the feedstock.
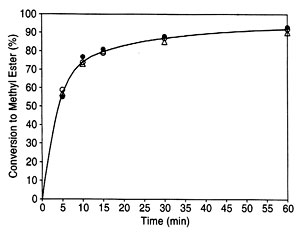
Fig. 2. Plot of turnover of soybean oil to methyl soyate in a transesterification determined by near infrared spectroscopy (O) and two NMR approaches 1H NMR spectroscopy (a-CH 2 and methyl ester protons) (•) and 1H NMR spectroscopy (glyceridic and methyl ester protons) (triangle) as described in the text. See Figure 1 for abbreviation.
Accordingly, the unconverted amount (in %) of vegetable oil, CTAG (defined by acyl groups), is given by Equation 2:
![]()
The conversion as determined by 1H NMR is related to the determination in wt% of residual triacylglyceride (vegetable oil) in vegetable oil methyl ester (biodiesel) by Equation 3:
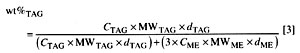
MW/TAG and MW/ME are the molecular weights of the vegetable oil and the corresponding methyl esters, respectively,. and dTAG and dME are the densities. Note that 3MW/ME = MW/TAG - 4.032 because of the difference in the number of protons as described above for Equation 1. As previously determined (25), the densities (at ambient temperature) of soybean oil and methyl soyate are 0.9092 and 0.87104, respectively. Slight variations in the fatty acid composition of the feedstock and the methyl esters can influence the determinations. Therefore, it is unreasonable to calculate several decimal places for MW. Note also that other experimental factors, such as accuracy of NMR integration, play a role. For the fatty acid composition of soybean oil, values given in the literature (33) were taken into account. That composition results in MW/TAG = 875. Thus 3MW/ME = 879. Taking these values into account, Equation 3 can be simplified (with rounding of all values, including density) to
![]()
Equation 4 can be modified for other biodiesel feedstocks, such as canola (rapeseed) oil, to take differing fatty acid compositions into account, although the differences are only minor. The differences will be greater for feedstocks with variations of the fatty acid chain length, for example, the short-chain tropical oils. The calculation of turnover of triacylglycerides to methyl esters in wt% is necessary because most contaminants in biodiesel standards are limited in wt%.
The previous NMR approach based on the methoxy protons of the methyl ester product and the a-CH2 protons utilizes Equation 5 (20) (modified to suit the present terminology):
![]()
The validity of the present approach correlating the spectra of samples drawn from a 6-L scale reaction was demonstrated by carrying out transesterification reactions and analyzing them by NIR and 1H NMR. Amounts of reactants were based on previous studies (34; see Experimental Procedures section), although other variations of the transesterification reaction exist (35, 36) and the original kinetics (37) have been questioned (35, 38). Obviously, the kind of reaction monitoring presented here will be possible, with appropriate minor modifications, for all variations of the transesterification reaction. A comparison of the three approaches shows that the yields are determined within a few percent by each of the approaches (Fig. 2). The approaches thus mutually confirm each other. However, trace contaminants at the low levels required by biodiesel standards, as discussed earlier for NIR, could not be fully analyzed by NMR either. On the other hand, the spectroscopic monitoring presented here could detect if the reaction takes an undesired course because the peaks of product methyl esters would either not increase or not increase to the expected intensity.
In conclusion, the present work has extended the previously reported (25) model system using fiber-optic NIR spectroscopy for transesterification reaction monitoring and biodiesel fuel quality assessment to samples obtained from an actual reaction. 1H NMR is also suitable for reaction monitoring and yield determination using the signals of the glyceridic and methyl ester protons besides the previously reported combination of methyl ester and a-CH2 protons (20). The NMR and NIR results can be correlated through simple equations and the validity of the methods mutually confirmed. NIR should remain the most attractive method because of ease, rapidity, and cost of analysis.
Acknowledgments
The author thanks Dale W. Ehmke for excellent technical assistance and Dr. David Weisleder for obtaining the NMR spectra.
References
1. Knothe, G., R.O. Dunn, and M.O. Bagby, Biodiesel: The Use of Vegetable Oils and Their Derivatives as Alternative Diesel Fuels, in ACS Symp. Ser. 666 (Fuels and Chemicalsfirom Biomass), American Chemical Society, Washington, DC, 1997, pp.172-208.
2. Dunn, R.O., G. Knothe, and M.O. Bagby, Recent Advances in the Development of Alternative Diesel Fuels from Vegetable Oils and Animal Fats, in Recent Res. Devel. Oil Chem. 1:31-56 (1997).
3. Raneses, A.R., L.K. Glaser, J.M. Price, and J.A Duffield, Potential Biodiesel Markets and Their Economic Effects on the Agricultural Sector of the United States, Ind. Crops Prod. 9:151-162 (1999).
4. Howell, S., U.S. Biodiesel Standards -- An Update of Current Activities, Soc. Automot. Eng. Technical Paper Ser. 971687, SAE, Warrendale, PA, 1997.
5. Mittelbach, M., Diesel Fuel Derived from Vegetable Oils, VI: Specifications and Quality Control of Biodlesel, Bioresource Technol. 56:7-11 (1996).
6. Freedman, B., W.F. Kwolek, and E.H. Pryde, Quantitation in the Analysis of Transesterified Soybean Oil by Capillary Gas Chromatography, J. Am. Oil Chem. Soc. 63:1370-1375 (1986).
7. Plank, C., and E. Lorbeer, Simultaneous Determination of Glycerol, and Mono-, Di- and Triglycerides in Vegetable Oil Methyl Esters by Capillary Gas Chromatography, J. Chromatogr. A 697:461-468 (1995).
8. Mittelbach, M., Diesel Fuel Derived from Vegetable Oils, V [1]: Gas Chromatographic Determination of Free Glycerol in Transesterified Vegetable Oi4s, Chromatographia 37:623-626 (1993).
9. Mittelbach, M., G. Roth, and A. Bergmann, Simultaneous Gas Chromatographic Determination of Methanol and Free Glycerol in Biodiesel, Ibid. 42:431-434 (1996).
10. Cvengros, J., and Z. Cvengrogová, Quality Control of Rapeseed Oil Methyl Esters by Determination of Acyl Conversion, J. Am. Oil Chem. Soc. 71:1349-1352 (1994).
11. Cvengrogová, Z., J. Cvengros, and M. Hronec, Rapeseed Oil Ethyl Esters as Alternative Fuels and Their Quality Control, Petroleum Coal 39:36-40 (1998).
12. Mariani, C., P. Bondioli, S. Venturini, and E. Fedeli, Vegetable Oil Derivatives as Diesel Fuel. Analytical Aspects. Note 1: Determination of Methyl Esters, Mono-, Di-, and Triglycerides, Riv. Ital. Sostanze Grasse 68:549-551 (1991).
13. Bondioli, P., C. Mariani, A. Lanzani, E. Fedeli, and S. Veronese, Vegetable Oil Derivatives as Diesel Fuel Substitutes. Analytical Aspects. Note 2: Determination of Free Glycerol, Ibid. 69:7-9 (1992).
14. Plank, C., E. Lorbeer, Minor Components in Vegetable Oil Methyl Esters 1: Sterols in Rape Seed Oil Methyl Ester, Fett Wiss. Technol. 96:379-386 (1994).
15. Plank, C., E. Lorbeer, On-Line Liquid Chromatography -- Gas Chromatography for the Analysis of Free and Esterified Sterols in Vegetable Oil Methyl Esters Used as Diesel Fuel Substitutes, J. Chromatogr. A 683:95-104 (1994).
16. Lechner, M., C. Bauer-Plank, and E. Lorbeer, Determination of Acylglycerols in Vegetable Oil Methyl Esters by On-Line Normal Phase LC-GC, J. High Resolut. Chromatogr. 20:581-585 (1997).
17. De Filippis, P., C. Giavarini, M. Scarsella, and M. Sorrentino, Transesterification Processes for Vegetable Oils: A Simple Control Method of Methyl Ester Content, J. Am. Oil Chem. Soc. 72:1399-1404 (1995).
18. Trathnigg, B., and M. Mittelbach, Analysis of Triglyceride Methanolysis Mixtures Using Isocratic HPLC with Density Detection, J. Liquid Chromatogr. 13:95-105 (1990).
19. Lozano, P., N. Chirat, J. Graille, and D. Pioch, Measurement of Free Glycerol in Biofuels, Fresenius J. Anal. Chem. 354: 319-322(1996).
20. Gelbard, G., 0. Brès, R.M. Vargas, F. Vielfaure, and U.F. Schuchardt, 1H Nuclear Magnetic Resonance Determination of the Yield of the Transesterification of Rapeseed Oil with Methanol, J. Am. Oil Chem. Soc. 72:1239-1241 (1995).
21. Daun, J.K., and P. Williams, Near Infrared Analysis of Oilseeds: Current Status and Future Directions, in New Techniques and Applications in Lipid Analysis, edited by R.E. McDonald and M.M. Mossoba, AOCS Press, Champaign, 1997, pp. 266-282.
22. Sato, T., Application of Near Infrared Spectroscopy for the Analysis of Fatty Acid Composition, Lipid Technol. 9:46-49 (1997).
23. Janosch, J., and S. Ebel, Bestimmung von Fettkennzahlen mit Hilfe der NIR-Spektrometrie, Pharmazie 48:824-828 (1993).
24. Sadeghi-Jorabchi, H., V.M.E. Wood, F. Jeffery, A. BrusterDavies, N. Loh, and D. Coombs, Estimation of Biodiesel in Lubricating Oil Using Fourier Transform Infrared Spectroscopy Combined with a Mid-Infrared Fiber-Optic Probe, Spectrosc. Eur. 6:16, 18, 20-21 (1994).
25. Knothe, G., Rapid Monitoring of Transesterification and Assessing Biodiesel Fuel Quality by NIR Spectroscopy Using a FiberOptic Probe, J. Am. Oil Chem. Soc. 76:795-800 (1999).
26. Mockel, W.D., and M.P. Thomas, Determination of Transesterification Reaction Endpoint Using NIR Spectroscopy, Proc. SPIE--Int. Soc. Opt. Eng. 1681:220-230 (1992).
27. Curtin, D.L., In-Process Analysis of Multifunctional Esters by NIR Spectroscopy, AT-Process 3:18-25 (1997).
28. Hansen, W.G., Shifting of -OH Absorption Bands on NIR Spectra of Esters, Appl. Spectrosc. 47:1623-1625 (1993).
29. Holman, R.T., and P.R. Edmondson, Near Infrared Spectra of Fatty Acids and Some Related Substances, Anal. Chem. 28:1533-1538 (1956).
30. McManis, G.E., and L.E. Gast, Near IR Spectra of Long Chain Vinyl Derivatives, J. Am. Oil Chem. Soc. 48:310-313 (197 1).
31. Murray, I., The NIR Spectra of Homologous Series of Organic Compounds, Near Infrared Diffuse Reflectance / Transm. Spectrosc., Proc. Int. NIR/NIT Conf., edited by J. Hollo, K.J. Kaffka, and J.L. Gonczy, Akad. Kiado, Budapest, 1986 (Publ. 1987), pp. 13-28.
32. Ozaki, Y., and Y. Liu, FT-NIR Spectroscopy of Some Long-Chain Fatty Acids and Alcohols, Macromol. Symp. 94:51-59 (1995).
33. The Lipid Handbook, 2nd edn., edited by F.D. Gunstone, J.L. Harwood, and F.B. Padley, Chapman & Hall, London, 1994, pp. 97-101.
34. Freedman, B., E.H. Pryde, and T.L. Mounts, Variables Affecting the Yields of Fatty Esters from Transesterified Vegetable Oils, J. Am. Oil Chem. Soc. 61:1638-1643 (1984).
35. Boocock, D.G.B., S.K. Konar, V. Mao, C. Lee, and S. Buligan, Fast Formation of High-Purity Methyl Esters from Vegetable Oils, Ibid. 75:1167-1172 (1998).
36. Boocock, D.G.B., S.K. Konar, V. Mao, and H. Sidi, Fast One-Phase Oil-Rich Processes for the Preparation of Vegetable Oil Methyl Esters, Biomass Bioenergy 11:43-50 (11996).
37. Freedman, B., R.O. Butterfield, and E.H. Pryde, Transesterification Kinetics of Soybean Oil, J. Am. Oil Chem. Soc. 63:1375-1380 (1986).
38. Noureddini, H., and D. Zhu, Kinetics of Transesterification of Soybean Oil, Ibid. 74:1457-1463 (1997).
[Received December 8, 1999; accepted March 11, 2000]
NIR Helps Turn Vegetable Oil into High-Quality Biofuel -- ARS News Release, June 15, 1999
Rapid Monitoring of Transesterification and Assessing Biodiesel Fuel Quality by Near-infrared Spectroscopy Using a Fiber-Optic Probe, by Gerhard Knothe, JAOCS 76, 795-800 (July 1999)
Monitoring a Progressing Transesterification Reaction by Fiber-Optic Near Infrared Spectroscopy with Correlation to 1H Nuclear Magnetic Resonance Spectroscopy, by Gerhard Knothe, JAOCS 77, 489-493 (May 2000)
Back to Biofuels Library Index
Biofuels
Biofuels Library
Biofuels supplies and suppliers
Biodiesel
Make your own biodiesel
Mike Pelly's recipe
Two-stage biodiesel process
FOOLPROOF biodiesel process
Biodiesel processors
Biodiesel in Hong Kong
Nitrogen Oxide emissions
Glycerine
Biodiesel resources on the Web
Do diesels have a future?
Vegetable oil yields and characteristics
Washing
Biodiesel and your vehicle
Food or fuel?
Straight vegetable oil as diesel fuel
Ethanol
Ethanol resources on the Web
Is ethanol energy-efficient?
